Abstract
Introduction Current treatment options for patients with relapsed or refractory (RR) lymphoma and multiple myeloma (MM) are limited, highlighting the unmet need for effective therapies in these disease settings. Purinostat Mesylate (PM) is a small molecule that is designed to selectively inhibit histone deacetylase (HDAC) enzymes class I and IIb, which are members of common oncogenic pathways in hematologic malignancies. This study examined PM monotherapy in patients with RR lymphoma and MM.
Methods This open-label, non-randomized, first-in-human, phase 1 two-center trial enrolled adult patients with lymphoma or MM who were refractory to or relapsed after ≥1 prior regimens. PM was administered by 30-minute intravenous infusion in a standard 3+3 dose escalation design. The trial was conducted in three stages sequentially as follows: the first stage as a single dose (Day 1), the second stage as multiple doses (Day 8,11, and 15), and the third stage as extended doses (Day1, 4, 8, 11 in 21-day cycles). The decision to proceed to the third stage is based on patient tolerability in the previous two stages, disease status, and continued benefit determined by investigators. Initially dosing started at 1.2 mg/m2, escalated to 2.4 mg/m2, 4.0 mg/m2, 6.0 mg/m2, 8.4 mg/m2, 11.2 mg/m2 and finally 15.0 mg/m2. Patients continued to receive PM until disease progression or other treatment discontinuation criteria were met. The primary objective was to determine the maximum-tolerated dose and recommended phase 2 dose (RP2D); secondary objectives were to assess the safety and tolerability, pharmacokinetic profiles, and preliminary anti-tumor activity. This ongoing trial is registered at Chinese Clinical Trial Registry, number ChiCTR20191121.
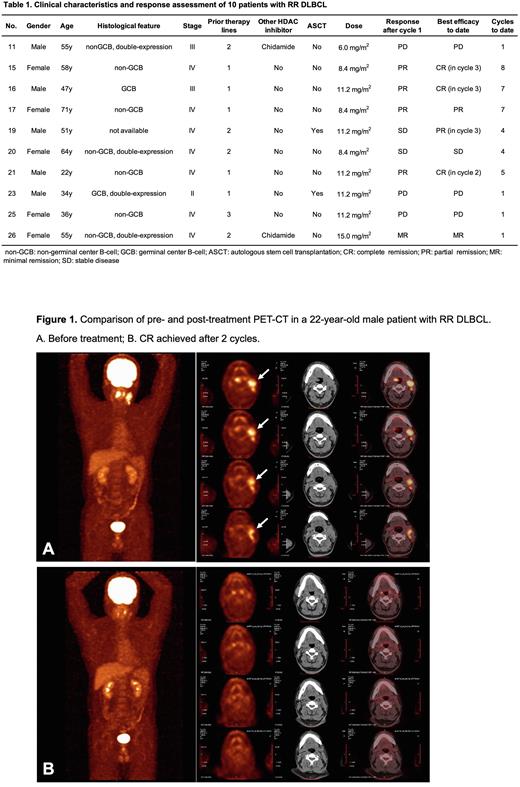
Results A total of 11 heavily pretreated MM patients(11/27,41%) and 16 lymphoma patients(16/27,59%) were enrolled to receive PM up to a maximum of 15.0 mg/m2 in the dose escalation. Most adverse events (AEs) were grade 1 to 2 and recovered with observation or symptomatic management. The most common Grade ≥3 AEs were thrombocytopenia (n=48.1%), neutropenia (n=48.1%), and anemia (11.1%), which occurred primarily during the extended dosing stages. AEs led to dose reductions in 4 patients. Dose limiting toxicities (DLTs) have not been observed. Twenty-seven patients were evaluable for responses. Eleven patients with RR MM had a disease control rate (DCR) of 72.7%. Eight patients had stable disease (SD) in cycle 1 and entered the third stage with a maximum of 6 extended cycles, of which 1 patient achieved a minimal remission (MR) in cycle 2. Nine out of 16 patients with RR lymphoma achieved objective responses rates (ORR) of 56.2%. Among them, six of 10 patients with diffuse large B-cell lymphoma (DLBCL) achieved objective responses (3 complete remissions [CR], 2 partial remissions [PR], and 1 MR). CRs occurred in 2 patients with DLBCL in cycle 3 (8.4mg/m2, 11.2 mg/m2) and 1 patient in cycle 2 (11.2 mg/m2). PRs occurred in 4 patient in cycle 1 (2 in 8.4 mg/m2, 2 in 11.2 mg/m2 ) and another patient who relapsed after 2 lines of prior therapy including autologous stem cell transplantation(ASCT) in cycle 3 (11.2 mg/m2). One MR and 1 SD have been observed in patients with double-expression DLBCL in cycle 1(15.0 mg/m2, 8.4 mg/m2) . Thus, the ORR in patients with RR DLBCL was 60.0% (6/10) and DCR was 70.0% (7/10). Two out of 4 follicular lymphoma patients relapsed after 2~4 lines of prior therapy also achieved objective responses (1 MR in cycle 1 and 1 PR in cycle 4). Of note, one patient with peripheral T-cell lymphoma who relapsed after four lines of treatment, including ASCT and Chidamide combination treatment with R-CHOP, obtained PR in cycle 1. Up to date, eight active response patients are still being treated. On the basis of the confirmed objective response and tolerability profile, the RP2D of PM was determined to be 8.4mg/m2 and 11.2mg/m2.
Conclusions PM has demonstrated tolerability and good anti-tumor activity across all dosing schedules studied, especially multiple objective responses and disease control in patients with DLBCL. These results support continued development of PM, alone or in combination with other therapies, for the treatment of RR DLBCL. An open-label, non-randomized, phase 2, multicenter trial will be soon conducted in China to evaluate the efficacy of PM monotherapy in RR DLBCL.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal